Subtitles & vocabulary
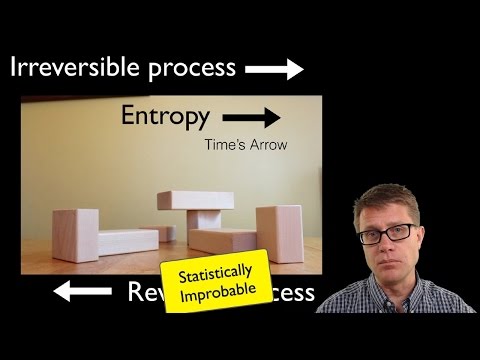
Second Law of Thermodynamics
00
李育真 posted on 2015/12/29Save
Video vocabulary
process
US /ˈprɑsˌɛs, ˈproˌsɛs/
・
UK /prə'ses/
- Transitive Verb
- To organize and use data in a computer
- To deal with official forms in the way required
- Noun (Countable/Uncountable)
- Dealing with official forms in the way required
- Set of changes that occur slowly and naturally
A2TOEIC
More time
US /taɪm/
・
UK /taɪm/
- Uncountable Noun
- Speed at which music is played; tempo
- Point as shown on a clock, e.g. 3 p.m
- Transitive Verb
- To check speed at which music is performed
- To choose a specific moment to do something
A1TOEIC
More amount
US /əˈmaʊnt/
・
UK /ə'maʊnt/
- Noun (Countable/Uncountable)
- Quantity of something
- Intransitive Verb
- To add up to a certain figure
A1TOEIC
More disorder
US /dɪsˈɔrdɚ/
・
UK /dɪs'ɔ:də(r)/
- Uncountable Noun
- State of confusion or a lack of organization
- Illness when the body is not functioning well
- Transitive Verb
- To disrupt the order or arrangement of something.
B2
More Use Energy
Unlock Vocabulary
Unlock pronunciation, explanations, and filters
